Vapreotide acetate salt is a synthetic octapeptide somatostatin analogue, widely used in the treatment of various conditions such as acromegaly, neuroendocrine tumors, and gastrointestinal bleeding. Its ability to inhibit the secretion of growth hormone and insulin-like growth factor-1 (IGF-1) makes it an essential drug in endocrinology. However, the extraction and purification of this compound require a meticulous approach due to its complex nature. In this blog post, SACH will share with you how to extract CAS No.: 103222-11-3 vapreotide acetate salt for sale.
Preparation and Safety Measures
Before beginning the extraction process, it is crucial to ensure that the laboratory is equipped with the necessary equipment and that all safety protocols are in place.
1. Laboratory Setup: Ensure that the laboratory is equipped with a fume hood, a refrigerated centrifuge, a lyophilizer, and a high-performance liquid chromatography (HPLC) system.
2. Personal Protective Equipment (PPE): Wear appropriate PPE including lab coats, gloves, and safety goggles.
3. Chemical Safety: Familiarize yourself with the Material Safety Data Sheet (MSDS) for all chemicals used in the extraction process.
Materials and Reagents
The following materials and reagents will be required for the extraction of vapreotide acetate salt:
- Vapreotide acetate salt starting material
- Acetonitrile (HPLC grade)
- Water (HPLC grade)
- Trifluoroacetic acid (TFA, HPLC grade)
- Diethyl ether
- Sodium chloride
- Sephadex G-25 column
- Reverse-phase C18 column
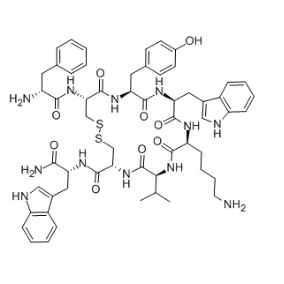
Extraction Process
The extraction of vapreotide acetate salt involves several steps, including dissolution, purification, and final drying.
1. Dissolution: Begin by dissolving the vapreotide acetate salt in a minimal volume of water. This step is crucial as it ensures that the compound is in a soluble state for subsequent purification steps.
2. Protein Precipitation: Add an equal volume of cold diethyl ether to the solution to precipitate any proteins that may be present. This step helps in reducing the complexity of the mixture and aids in the selective extraction of vapreotide.
3. Centrifugation: Centrifuge the mixture at 4°C for 15 minutes at a high speed (e.g., 10,000 rpm) to separate the organic and aqueous layers.
4. Liquid-Liquid Extraction: Carefully remove the organic layer and repeat the extraction process until no more vapreotide is extracted into the organic phase. This can be monitored using a UV-Vis spectrophotometer.
5. Evaporation: Evaporate the organic solvent under reduced pressure using a rotary evaporator. The residue should be a crude extract of vapreotide acetate salt.
6. Gel Filtration: Purify the crude extract using a Sephadex G-25 column. This step helps in removing any small molecular weight impurities.
7. Reverse-Phase HPLC: Load the purified extract onto a reverse-phase C18 column and perform HPLC with a gradient elution using a mixture of acetonitrile and water containing 0.1% TFA.
8. Fraction Collection: Collect the fractions containing vapreotide acetate salt based on their retention time and UV absorbance.
9. Lyophilization: Freeze-dry the collected fractions to obtain the pure vapreotide acetate salt as a solid.
Quality Control
After extraction, it is essential to perform quality control checks to ensure the purity and integrity of the vapreotide acetate salt.
1. HPLC Analysis: Analyze the purity of the extracted vapreotide acetate salt using HPLC. The purity should be greater than 95%.
2. Mass Spectrometry: Confirm the molecular weight and identity of the compound using mass spectrometry.
3. Nuclear Magnetic Resonance (NMR): Perform NMR spectroscopy to confirm the structure of vapreotide acetate salt.
4. Biological Activity Assay: Test the biological activity of the extracted vapreotide acetate salt using appropriate cell-based assays.
Conclusion
The extraction of vapreotide acetate salt is a complex process that requires careful planning and execution. By following the steps outlined in this guide, researchers can successfully extract and purify this important somatostatin analogue. It is important to note that the extraction process should be carried out in a controlled laboratory environment with strict adherence to safety protocols. The final product should be of high purity and biological activity, ensuring its efficacy in clinical applications.
https://www.hzsqchem.com/How-to-extract-CAS-No-103222-11-3-vapreotide-acetate-salt.html
SACH
sales@hzsqchem.com